Lecture Series on BioChemistry I by Prof.S.Dasgupta, Dept of Chemistry, IIT Kharagpur. For more details on NPTEl visit http://nptel.iitm.ac.in
Tuesday, November 30, 2010
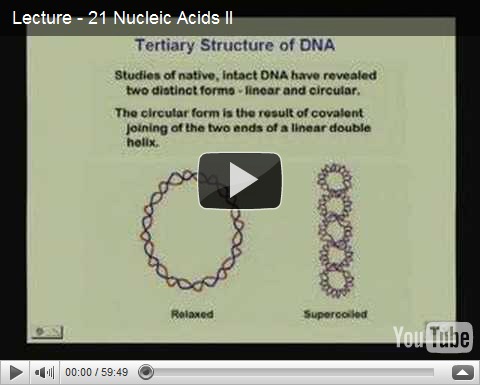
Nucleic Acids Lecture III
Labels: Biomolecules, Biomolecules video lecture, Lectures, Nucleic Acids, Video lectures
Nucleic Acids Lecture II
Labels: Biomolecules, Biomolecules video lecture, Lectures, Nucleic Acids, Video lectures
Lecture Series on BioChemistry I by Prof.S.Dasgupta, Dept of Chemistry, IIT Kharagpur. For more details on NPTEl visit http://nptel.iitm.ac.in
Monday, November 29, 2010
Nucleic Acids Lecture I
Labels: Biomolecules, Biomolecules video lecture, Lectures, Nucleic Acids, Video lectures
Lecture Series on BioChemistry I by Prof.S.Dasgupta, Dept of Chemistry, IIT Kharagpur. For more details on NPTEl visit http://nptel.iitm.ac.in
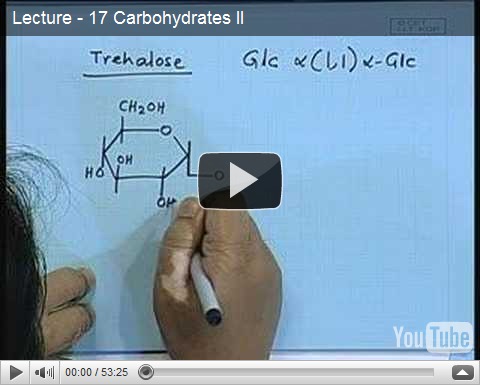
Carbohydrate Lecture II
Labels: Biomolecules, Biomolecules video lecture, Carbohydrates, Lectures, Video lectures
Lecture Series on BioChemistry I by Prof.S.Dasgupta, Dept of Chemistry, IIT Kharagpur. For more details on NPTEl visit http://nptel.iitm.ac.in
Carbohydrates lecture I
Labels: Biomolecules, Biomolecules video lecture, Carbohydrates, Lectures, Video lectures
Lecture Series on BioChemistry I by Prof.S.Dasgupta, Dept of Chemistry, IIT Kharagpur. For more details on NPTEl visit http://nptel.iitm.ac.in
Tuesday, November 23, 2010
Lecture Video - 3 Enzymes as Biocatalysts
Labels: Biomolecules, Biomolecules video lecture, Enzymology, Lectures, Video lectures
Monday, November 22, 2010
Lecture Video Enzymes II
Labels: Biomolecules, Biomolecules video lecture, Enzymology, Lectures, Video lectures
What is Enzymes?
Enzymes are biological catalysts: this means that they speed up the chemical reactions in living things. Without enzymes, our guts would take weeks and weeks to digest our food, our muscles, nerves and bones would not work properly and so on - we would not be living!
A catalyst is any substance which makes a chemical reaction go faster, without itself being changed. A catalyst can be used over and over again in a chemical reaction: it does not get used up. Enzymes are very much the same except that they are easily denatured (destroyed: but do NOT use this word since the protein molecule is not broken down into amino-acids, it just loses it shape and will not work any more) by heat. Our enzymes work best at body temperature. Our enzymes also have to have the correct pH.
All enzymes are made of protein; that is why they are sensitive to heat, pH and heavy metal ions. Unlike ordinary catalysts, they are specific to one chemical reaction. An ordinary catalyst may be used for several different chemical reactions, but an enzyme only works for one specific reaction.
Human saliva contains an enzyme called amylase. This enzyme helps to turn starch into a sugar called maltose. When you swallow a mouthful of food, the amylase stops working because it is much too acid in the stomach pH 2. Amyalse works best in neutral or slightly alkaline conditions, i.e. at about pH 7. When your food gets into the small intestine, more amylase is made by the pancreas and this turns the remaining starch into maltose. Another enzyme (maltase) turns all this maltose into glucose. Glucose is then absorbed into the blood.
Enzymes in the human alimentary canal and what they digest:
Enzyme Substrate
Amylase Starch
Maltase Maltose
Sucrase Sucrose
Lipase Fats
Pepsin Proteins
All animals, green plants, fungi and bacteria produce enzymes: so enzymes are not just about digesting food. The enzymes which we use to digest our food are extra-cellular, that means they are found outside cells. We also have enzymes inside our cells; these are intra-cellular enzymes. Enzymes are used in ALL chemical reactions in living things; this includes respiration, photosynthesis, movement growth, getting rid of toxic chemicals in the liver and so on.
Viruses are rather different, but you do not need to know much about them for GCSE, so just make sure that you don't catch any!
Enzymes must have the correct shape to do their job. They are made of proteins, and proteins are very easily affected by heat, pH and heavy metal ions. Some people say that enzymes work like a key in a lock. If the key has been twisted by heat, or dissolved in acid or stuck up with chewing gum it will not work. Enzymes change their shape if the temperature or pH changes, so they have to have the right conditions. Copper ions are poisonous: if you get copper ions in your blood they will block up some of the important enzymes in red and white blood cells.
See the Video Lectures on Enzymes
Enzymes Part II
Enzymes Part III : Enzymes as Biocatalysts
Enzymes Part IV
Enzyme Part V : Specificity of Enzyme Action
Enzyme Part VI : Kinetics of Enzyme Catalysed Reactions
Enzyme Part VII
Enzyme Part VIII
Lecture Video Enzymes I
Labels: Biomolecules, Biomolecules video lecture, Lectures, Video lectures
Sunday, November 21, 2010
Video Lecture : Protein Structure–I
Labels: Biomolecules, Biomolecules video lecture, Lectures, Video lectures
Saturday, November 20, 2010
Video Lecture : Protein Structure 4
Labels: Biomolecules, Biomolecules video lecture, Lectures, Video lectures
Lecture Series on BioChemistry I by Prof.S.Dasgupta, Dept of Chemistry, IIT Kharagpur.
Video Lecture : Protein structure II
Labels: Biomolecules, Biomolecules video lecture, Lectures, Video lectures
Video Lecture : Protein Structure III
Labels: Biomolecules, Biomolecules video lecture, Lectures, Proteins, Video lectures
Friday, November 19, 2010
Protein is an essential nutrient
Protein is one of the nutrients along with carbohydrate, fat, vitamins, minerals, and water. The source of all of these nutrients is good. Some foods contain much higher amounts of specific nutrients than others and sometimes we refer to certain foods as “protein foods”.
It is important to realize that all goods contain more than one nutrient and most foods contain substantial amounts of several nutrients. For example, meat, which is a good source of protein, carbohydrate, fat, riboflavin and calcium.
Protein is an essential nutrient. There is no life without protein. Protein is contained in every part of your body, the skin, muscles, hair, blood, body organs, eyes, even fingernails and bone. Next to water, protein is the most plentiful substance in your body.
Structure of Proteins
Proteins are composed of small units. These units are the amino acids which are called the building blocks of protein. There are about 20 different amino acids which are commonly known.
Each different protein is composed of various amino acids put together in varying order with almost limitless combinations. Most proteins are large molecules that may contain
several hundred amino acids arranged in branches and chains.
Functions
Protein has a critical physiological function. Protein is primarily used in the body to build, maintain, and repair body tissues. In the event that protein intake is greater than that required by the body for this primary function, excessive protein is converted to energy for immediate use or stored in the body as fat. Protein energy will be used only after other energy sources (carbohydrate and fat) are exhausted or unavailable.
See the Video, given by
Lecture Series on BioChemistry I by Prof.S.Dasgupta, Dept of Chemistry, IIT Kharagpur.
Lecture Video I
Lecture Video II
Lecture Video III
Lecture Video IV
Lecture - 2 Amino Acids II
Labels: Biomolecules, Biomolecules video lecture, Lectures, Video lectures
Amino Acids Notes part1
Amino acids are monomeric units of proteins. Proteins are high molecular weight organic polymer. Although about 200 to 300 amino acids occur in nature, only 20 of them are seen in human body.
Amino acids are relatively simple molecules containing both an amine group and an acid group. The biologically important amino acids are the alpha-amino acids that have the amine and acid groups attached to the same carbon atom. There are more than 300 known natural amino acids; however, only 20 of them are used in protein synthesis. Francis Crick (who with James Watson determined the structure of DNA) labeled this set of amino acids the magic 20. Other amino acids are found in certain proteins, but in almost all cases these additional amino acids result from the modification of one of the magic 20 after the protein formed.
Definition:
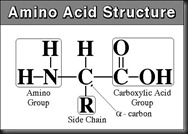
“A substance which has both carboxyl and amino group in the same molecule is called ‘amino acid’. The amino group is basic while the carboxyl group (-COOH) is acidic in nature”.
Amino acids are a group of organic compounds containing two functional groups – amino and carboxyl. The amino group (-NH2) is basic while the carboxyl group (-COOH) is acidic in nature.
All proteins are polymers of amino acids and all these acids except two have an amino group attached to the carbon atom next to the carboxyl group. The general formula of the amino acid is ..
Rules to write the Amino acids:
We should write the general formula by using the given points:
- We should write the Carboxyl group on Top side of the center carbon (asymmetric carbon)
- We should write the Amino group on Left side of the centre carbon (asymmetric carbon).
- We should write the hydrogen atom on Right side of the centre carbon (asymmetric carbon).
- We should write the ‘R’ side chain at the Bottom of the centre carbon (asymmetric carbon).
The amino acids are termed as α-amino acids, if both the carboxyl and amino groups are attached to the same carbon atom. The amino acids mostly exist in the ionized form in the biological system.
Stereochemistry:
 Carbon atom is attached to four different groups, which is simply called “asymmetric” and therefore exhibits optical isomerism. All amino acids contain asymmetric carbon, except Glycine. (Glycine is Optically inactive compound).
Carbon atom is attached to four different groups, which is simply called “asymmetric” and therefore exhibits optical isomerism. All amino acids contain asymmetric carbon, except Glycine. (Glycine is Optically inactive compound).
D & L Forms:
Amino acids can be shown in two different forms depends on the position of amino group on the asymmetric carbon atom. When the amino group situated on the left side, which is L-form. When the amino group situated on the sight side, which is D-form. All naturally occurring amino acids are in L-configuration.
See the Video Lecture here.
See the Video lecture Just Click Here
(Just click on the Image)
Lecture Series on BioChemistry I by Prof.S.Dasgupta, Dept of Chemistry, IIT Kharagpur.
Lecture - 1 Amino Acids I
Labels: Biomolecules, Biomolecules video lecture, Lectures, Video lectures